The developement of the early Atomic Theory first began with the Greeks in 300 BC with a man called Democritus. He said that atoms are indivisible particles. This is the first mention of atomos, also known as atoms! Democritus theory was not testable, but only a conceptual model. It had no mention of any atomic nucleus of its constituents. It could not be used to explain chemical reactions. Thus, this theory was accepted for about 3000 years.
Lavoisier: ( late 1700s)
This man came up with the law of conservation of mass and law of definite proportions. It is said his concepts was not considered to be an Atomic Theory because it didn't discuss what atoms were or how they were arranged.
Proust: (1799)
Proust said if a compound is broken down into its constituents, the products exist in the same ratio as in the compound. This theory experimentally proved Lavoisier's Law.Dalton: (early 1800s)
Dalton considered atoms as solid indestructible spheres, like billiards. The problem with Dalton's theory is that it didn't mention subatomic particles, explain isotopes, and had no mention of the nucleus.
J.J Thomson: (1850s)
This man came up with the Raison Bun Model. It showed solid, positive spheres with negative particles embedded in them. It was the first atomic theory to have positive and negative charges! It introduced the idea of the nucleus. The problem with this theory is that it no mention of neutrons, therefore, radioactive decay cannot be explained. it also doesn't explain how electrons exist outside the nucleus and didn't explain electrons role in chemical bonding.
Rutherford: (1905)
Rutherford showed that atoms have a positive dense center with electrons outside it. this resulted in the Planetary Model which explains why electrons spin around the nucleus. it suggest atoms are mostly empty space. But, it showed instability because the negative and positive should attract and destroy the atom. Thus, it had not mention of neutrons and did not explain valence level electons role in chemical bonding.
Bohr: (1920s)
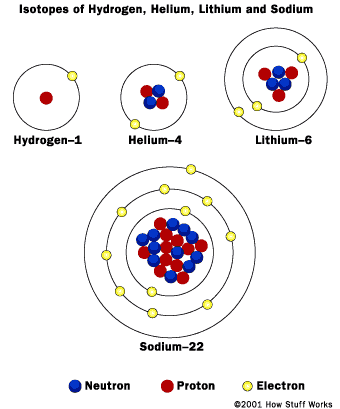
Bohr said the electrons must only exist in specific orbitals around the nucleus. It explains how valence electrons are involved in bonding and the difference between ionic and covalent bonding. It resolved the problem of atomic instability and the atomic spectra. It also includes the neutron


No comments:
Post a Comment